
Futureco Bioscience, a global leader in sustainable agricultural solutions, today announced a groundbreaking advance in microbiological quality control for biological insecticides. By developing the first ISO 17025-certified method for viable spore quantification using automated particle counting technology, the company is setting a new industry benchmark for accuracy, speed, and reliability. This milestone reinforces Futureco Bioscience’s commitment to scientific innovation, regulatory excellence, and delivering trusted biological solutions to farmers worldwide.
Read the Full Press Release Below –
NOFLY® Changes the Rules of Quality Control in Biological Insecticides
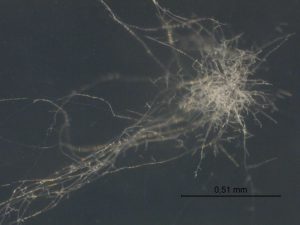
Early-stage colony of Cordyceps fumosorosea FE9901 grown on potato dextrose agar at 24 °C. Photo: Lola Pinyol, researcher, Microbiology Unit, Futureco Bioscience
Developed by Futureco Bioscience to ensure faster and more accurate microbiological quality control of NOFLY®, the new ISO 17025-certified method marks a significant advance in the standardization of viable spore quantification for biological insecticides.
Olérdola (Barcelona), September 2nd, 2025 – Futureco Bioscience has achieved a new milestone in the quality control of biological crop protection products with the development and ISO 17025-accredited validation of a new quantification method for viable spores in the company’s flagship bioinsecticide NOFLY®. The method is the first certified under ISO 17025 standards using automated particle counting technology for viable spore quantification.
This achievement follows the recent accreditation expansion (July 2025), where the Spanish National Accreditation Body (ENAC) recognized Futureco Bioscience’s Analytical Services Department (ASD) for two advanced analytical procedures. Today’s announcement puts a spotlight on the microbiological innovation behind that expansion, with NOFLY® leading the way.
“This is not just an internal validation. It’s a certified, ISO-accredited protocol for the entire biological control input industry,” said Belén López García, Director of Analytical Services. “We’re setting a new benchmark in how biopesticides like NOFLY® are measured, validated, and trusted by regulators, distributors, and farmers.”
From Petri Dishes to Fluorescent Microscopy: Revolutionizing Viable Spore Quantification
NOFLY® is widely recognized for its efficacy against whiteflies, thrips, aphids, and other major insect pests. Until now, its microbiological quality control required manual counting of colony-forming units (CFUs) on agar plates: a process that took 2–3 days and involved significant variability and labor.
The new ISO-certified method uses the LUNA™ FX-7 automated particle counter and a fluorescent viability staining to detect and quantify live spores in minutes, providing consistent, reproducible results across technical-grade and formulated products.

Juan González, researcher, Molecular Biology Unit, Futureco Bioscience.
Developed by an expert team in the Molecular Biology and Microbiology Units of Futureco Bioscience’s R&D Department, the method reflects both scientific rigor and practical expertise. “It brings speed, precision, and traceability to a process that was traditionally slow and prone to underestimation,” explained Juan Bautista González López, lead scientist.
Accuracy, Repeatability, and Reliability Backed by Data
The method was validated over two years using commercial batches of NOFLY® and its technical-grade active ingredient. It was benchmarked against certified reference materials, showing high accuracy (103% recovery rate), low standard deviation, and excellent reproducibility across operators and days. These metrics surpass conventional CFU-based counting.

Bioball™ CRM with viable Candida albicans ATCC 10231 (1×10^8 cells per Bioball), used to assess method accuracy.
It also confirms long-term batch stability and verifies that co-formulants in the final product do not interfere with viable spore detection. This ensures consistent performance from production to application.
A Quality Milestone for NOFLY® and the Biologicals Industry
With this certification, NOFLY® becomes the first fungal biopesticide with a viable spore quantification method based on automated particle counting validated under ISO 17025.
“This is a strategic move for Futureco Bioscience and for all our partners in the field,” stated Rafael Juncosa, CEO and President. “NOFLY® has always been a trusted biological solution for sustainable pest control. Now, it also leads the industry in analytical traceability and regulatory compliance.”
The development also paves the way for greater acceptance of biocontrol solutions in high-value export markets, where quality control and documentation are under increased scrutiny.
About NOFLY®
NOFLY® is a biological insecticide developed by Futureco Bioscience based on Cordyceps fumosorosea (formerly Paecilomyces fumosoroseus) FE9901. Registered and commercialized in multiple countries, it offers sustainable and effective control of pests like whiteflies, aphids, and thrips in protected and open-field crops. Its formulation, quality, and efficacy are backed by extensive R&D and now by an industry-first certified viable spore quantification method.
For further information, technical sheets or distributor queries, visit www.futurecobioscience.com or contact info@futurecobioscience.com.

Leave a Reply